Beijing Rishang Security Door was founded in 1996 and is headquartered in the East District of Beijing Economic and Technological Development Zone, integrating R & D, manufacturing, marketing and after-sales service. It is a large-scale production enterprise led by the development and manufacture of door products such as Rishang anti-theft doors , Rishang fire doors, and fire shutter doors. The company has strong economic strength, advanced technology and equipment, high brand awareness, diversified and serialized products, and is one of the leading companies in the industry.

Beijing Rishang Security Door has been independently researched and developed, with innovative breakthroughs, and its production technology has reached the domestic advanced level. Existing products, anti-theft doors, steel armored doors, steel carved doors, ventilation doors, stainless steel doors, building electrical control doors, steel fire doors, fire shutter doors and other door products: In 2011, we independently developed and produced steel column columns, copper and aluminum Composite wing type, bathroom type and other series radiator products: intelligent monitoring electronic security products and other types, hundreds of models. The products strictly implement national industry and local standards, adopt high-quality materials, and have exquisite processing technology. The R & D center was established in 2004, the CNC machining center was established in 2006, and the experimental center was expanded in 2008. It has obtained a number of national patents and became a national standard participating unit including the multi-functional portal, and a standing director unit of the China Construction Metal Structure Association.

Over the years , Rishang Security Door Group has been devoted to building the industry's first-class brand, and sincerely rewarding consumers with the highest quality products and the most satisfactory service. It is a well-known reputation brand in Beijing, and also the first choice for real estate, builders and decoration companies. The China Engineering Construction Standardization Association has been awarded "Recommended Products", and the company has won many honors including "ISO9001 Certified Enterprise", "Fire Product Type Approved Enterprise", "Quality Trusted Unit" and "Designated Manufacturing Enterprise".

The above introduction about Rishang Security Door Group, I hope that through this article, you can have a better understanding of Rishang Security Door . If you have any questions, you can leave a message at the bottom of the page, we will answer you as soon as possible. Thank you for your support of this website, and related articles are published one after another, please continue to check.
What, the decoration still uses his own money? ! The Qi family is decorated in installments, with an ultra-low annual interest rate of 3.55% and a maximum loan of 1 million. Apply now to enjoy the discount
If you are interested in brand cooperation, content cooperation, and advertising of this website, please send an email to :.
Anti-theft door anti-theft door brand
Description about Coronavirus(COVID-19)test Test
The Coronavirus(COVID-19)test is a solid phase immunochromatographic assay used in the rapid, qualitative and differential detection of IgG and IgM antibodies to the 2019 novel coronavirus in human whole blood, serum or plasma. The Coronavirus(COVID-19)test is intended for professionals within highly complex settings and or pharmacies. The test delivers rapid results between 2 and 10 minutes from individuals having signs and symptoms of SARS-CoV-2 infection
What conditions to do the Coronavirus(COVID-19)test?
1.Breating difficulty
2.skin sores, which may become a skin ulcer that heals very slowly
3.stuffy nose, runny nose, and nosebledds.
4.swallowing difficulty
TEST OVERVIEW for the Coronavirus(COVID-19)test
- Utilizes human whole blood, serum, or plasma
- Used in rapid, qualitative and differential detection of IgG and IgM antibodies
- Delivers clinical results between 2 and 10 minutes at the point-of-care
- Visual interpretation of results
- No special equipment needed
PERFORMANCE CHARACTERISTICS
The COVID-19 lgG/lgM Rapid Test (Whole Blood/Serum/Plasma) has been evaluated with the 126 samples obtained from patients exhibiting pneumonia or respiratory symptoms. The results were compared to Fluorescent Real Time Polymerase Chain Reaction (RT-PCR) or clinical diagnosis (including chest Computed Tomography and clinical signs etc.) of [Diagnosis and treatment of novel coronavirus pneumonia."
IgG/IgM Rapid Test results compared to SARS-CoV-2 RT-PCR results:
| COVID-19 RT-PCR Assay | Coronavirus(COVID-19)test | ||
| Positive | Negative | Total | |
| Positive | 103 | 9 | 112 |
| Negative | 0 | 14 | 14 |
| Total | 103 | 23 | 126 |
| 95% CI | |||
| Sensitivity | 103/112 | 91.9% | (85.3% – 96.3%) |
| Specificity | 14/14 | 100% | (76.8% – 100%) |
| Overall | 117/126 | 92.8% | (86.9% – 96.7%) |
The sensitivity of lgM only test is 89.2% (100/112) and specificity is 100% (14/14).
The sensitivity of lgG & IgM test is 91.9% (103/112) and specificity is 100% (14/14).
RT-PCR Method testing was included as clinical site method. Nucleic acid was detected by probe fluorescence PCR. This product contained primers and probes (ORF1ab gene and N gene strains of coronavirus) and internal controls (housekeeping gene beta Globin gene sequences) used in RT-PCR for the in vitro qualitative detection of SARS-CoV-2 RNA in nasopharyngeal swab specimens. The novel coronavirus2019-ncov specific probe was respectively labeled with FAM fluorescence and ROX fluorescence, and the internal standard gene was labeled with VIC fluorescence. The minimum detection limit of the fluorescent RT-PCR assay is 10 copies/reaction.
LIMITATIONS
- Use fresh samples only.
- This test has not been reviewed by the FDA.
- Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus.
- Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals.
- Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
- Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E. Some specimens containing unusually high titer of heterophile antibodies or rheumatoid factor may affect expected results.
-
Not for the screening of donated blood
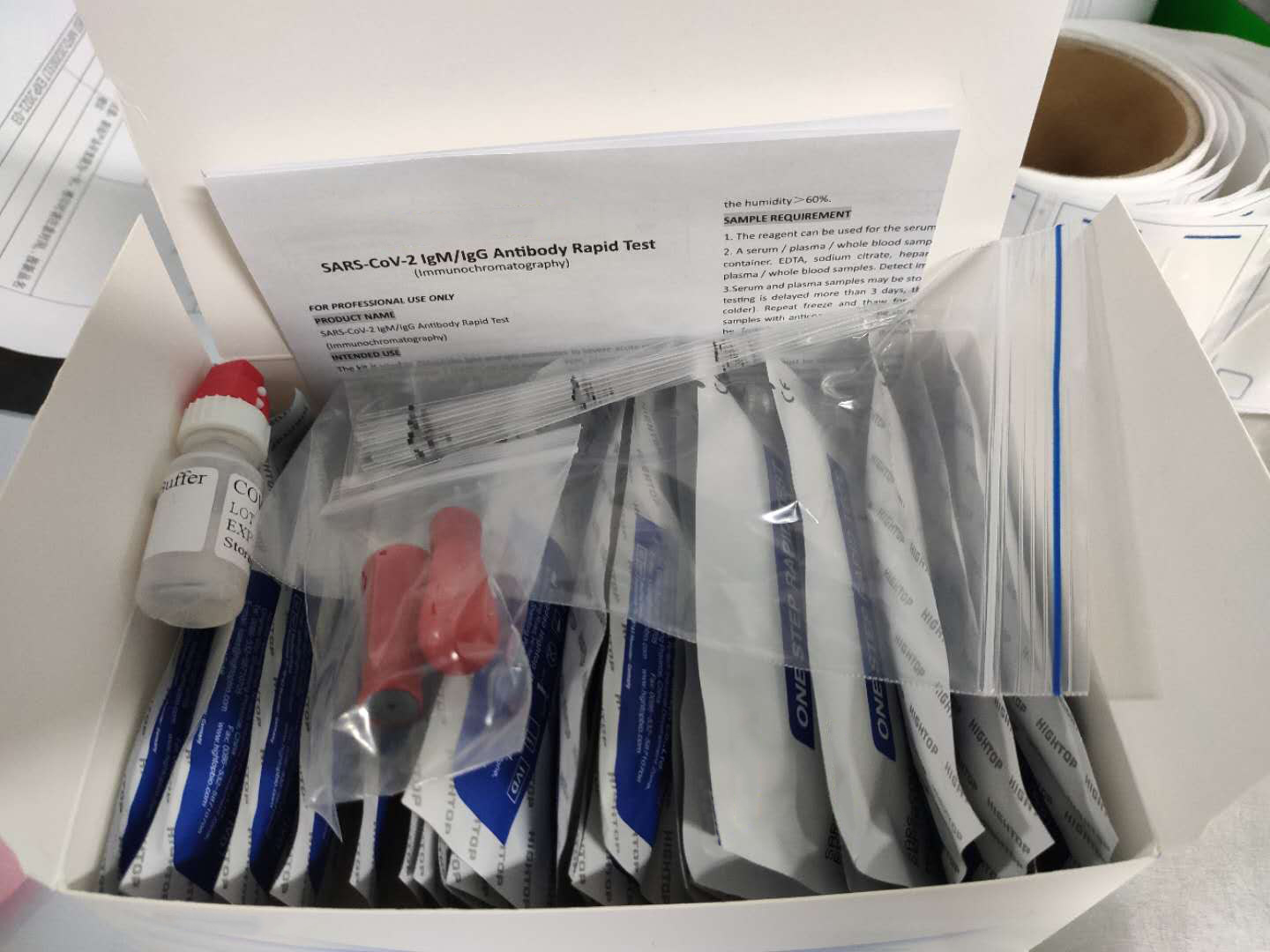
Rapid Test,Coronavirus Test Kit,Coronavirus Test,Covid-19 Test
Dongguan Smart Furniture Co.,Ltd , https://www.smtfurniture.com