Separation of active enantiomeric metabolites of risperidone by UPC2/MS/MS and used to detect metabolic stability
Jennifer Simeone, Yun Alelyunas, Mark Wrona, and Paul Rainville
background
Many drug candidates and their metabolites contain one or more chiral centers. The identification and monitoring of possible various enantiomers is a crucial step in the drug development process. As we all know, Supercritical Fluid Chromatography (SFC) is an efficient technique for chiral separation. In addition, SFC also has many advantages, such as high analysis efficiency, fast separation speed, and compatibility of the used solvent and MS.
solution
In this technical brief we show the use of UPC2®/MS/MS for the enantiomeric separation and detection of the active metabolite of risperidone, 9-hydroxyrisperidone. The researchers used a 9-hydroxy risperidone standard for method optimization and then applied it to a sample of risperidone incubated in human liver microsomes. The experiment monitored the conversion from the parent compound to the hydroxy metabolite.
This method was used to analyze incubated samples quenched at t, 0, 15, 30, 60, 90, and 120 min to monitor the conversion from risperidone to 9-hydroxyl metabolites. Figure 2 shows an example injection where the two expected peaks for the 9-hydroxy metabolite are located at 3.92 and 4.29 min, respectively. However, two additional peaks appeared at 4.48 and 4.76 min, which may be a few reported 7-hydroxyrisperidone metabolites. 1
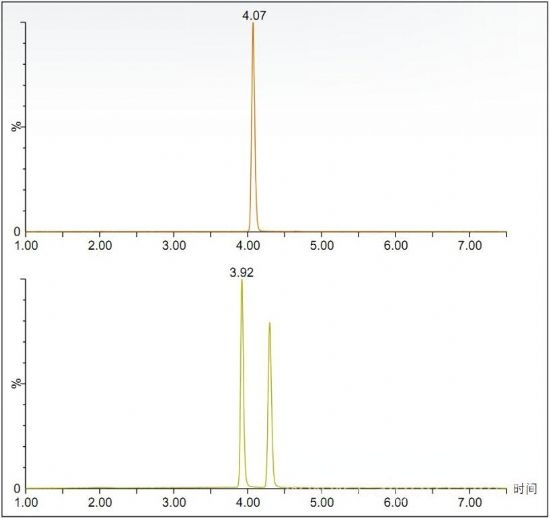
Figure 1. Risperidone (top) and R/S 9-hydroxyrisperidone (bottom).
Figure 1 shows the achiral risperidone and 9-hydroxyrisperidone obtained with Waters® Trefoil CEL2 3×150 mm, 2.5 μm columns and ammonium formate modified methanol as a cosolvent. The optimized chromatograms for the R and S configurations.
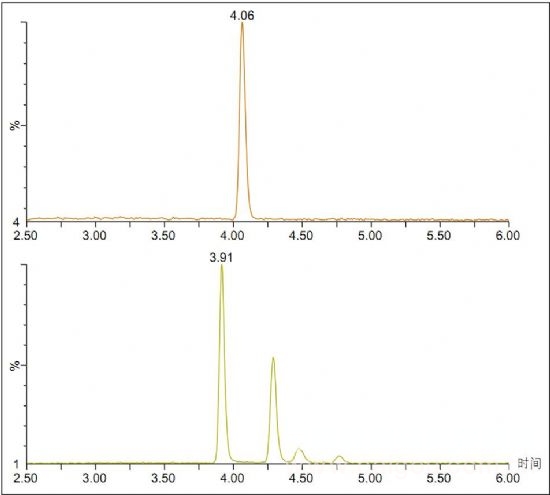
Figure 2. Analysis of risperidone (top) and hydroxyrisperidone (bottom) incubated with risperidone microsomes at t = 120.
We plotted the peak areas of the parent compounds, risperidone, and the four hydroxy metabolites obtained as a function of time. As shown in Figure 3, most of the parent compounds were converted to metabolites within 30 minutes. Although the R and S configurations cannot be absolutely confirmed, according to published data2, the process is more likely to form R-9-hydroxyrisperidone. Therefore, we speculate that the earlier eluting peak is in the R configuration and the second peak is in the S configuration.

Figure 3. Measured peak area-time profiles of incubated parent compounds (risperidone) and hydroxy metabolites quenched after 15, 30, 60, 90, and 120 min, respectively.
to sum up
The combination of UPC2 and MS detection facilitates the enantiomeric separation of 9-hydroxyrisperidone, the active metabolite of risperidone. Separating different chiral configurations of compounds is a crucial step in drug development. In addition, we have also demonstrated that converging chromatography can be successfully applied to metabolic stability studies.
references
1. G Mannens, ML Huang, W Meuldermans, J Hendirckx, R Woestenborghs, J Heykants. Absorption, metabolism, and excretion of risperidone in humans. Drug Metab Dispos 21: 1134-1141, 1993.
2. N Yasui-Furukori, M Hidestrand, E Spina, G Facciola, MG Scordo, G Tybring. Different Enantioselective 9-Hydroxylation of Risperidone by the Two Human CYP2D6 and CYP3A4 Enzymes. Drug Metab Dispos 29: 1263-1268, 2001.
Download the complete program please click zh_ using UPC2MSMS on the separation of the active metabolites of risperidone and its application in metabolic stability monitoring.pdf